Effluent or Water Treatment Processes (WTP/ETP)
 Criteria
of Water: Criteria are requirements that a water source must satisfy
in order to be used for a particular purpose. In other terms, criteria are specifications
indicating the minimum quality level that the water must have to support a predefined
use. Criteria are important in defining the characteristics of inlet water streams.
Criteria
of Water: Criteria are requirements that a water source must satisfy
in order to be used for a particular purpose. In other terms, criteria are specifications
indicating the minimum quality level that the water must have to support a predefined
use. Criteria are important in defining the characteristics of inlet water streams.Different criteria exist for different water uses:
Water
Use
Water
Quality Criteria
Drinking Water : Microbial count, pH, color, turbidity,Organic content, pH, toxic compounds,dissolved oxygen, Temperature, Salt, Metals, etc.
Industrial : pH, suspended solids, temperature, dissolved oxygen.
Swimming,
fishing : Similar to drinking
water criteria but not as stringent.
Cooling, navigation : Floating solids, suspended solids and pH.
Cooling, navigation : Floating solids, suspended solids and pH.
Standards of Water: Standards
are the characteristics that the waste water must satisfy to be lawfully discharged
to a receiving body of water or to a POTW. In other terms standards refer to
the quality of the outlet
water, after its intended use.
Criteria vs. Standards of
water:
Types of Discharges of
Industrial Wastewaters:
1. Direct industrial
discharges to freshwater or saltwater streams.
2. Industrial discharges to a
Publicly Owned Treatment Works (POTW).
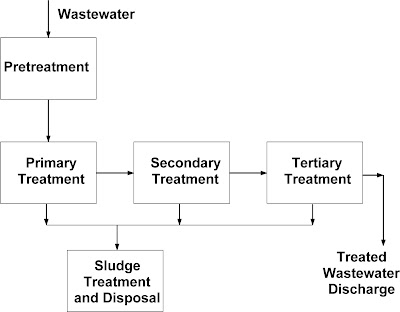
Classification of
Wastewater Treatments Processes:
Pretreatment: Pretreatment usually
refers to any treatment the wastewater is subjected to before entering a conventional wastewater treatment plant, such as a POTW. Pretreatment typically involves operations connected to
separation of very coarse or easily
Pretreatment of industrial
wastewaters commonly refers to any treatment required to make the water
acceptable for discharge to a POTW.
It is common practice to classify wastewater treatment
processes in three categories:
i) Primary treatment: Primary treatment pertains to
the removal of easily separable materials such as oils, floating solids, or quickly
settling solids, and the preparation of the
ii) Secondary treatment: Secondary treatment is
typically the most important part of the process, and is used primarily to
remove the bulk of the suspended solids, organic materials (both hazardous and
non-hazardous), and other soluble materials. Biological treatment constitutes
the process of choice during secondary treatment of wastewater.
iii) Tertiary treatment: Tertiary treatment, involving
processes such as sand filtration,
reverse osmosis, adsorption,
and electro dialysis, is used (if necessary) to remove any
Sludge
treatment and disposal: Sludge treatment and disposal includes all the operations
connected with the concentration, stabilization, and final disposal of the
semi-solid sludge produced during the primary, secondary and tertiary
treatments. Sludge treatment and disposal includes operations such as gravity
thickening, air flotation, aerobic and anaerobic digestion, chemical or heat
stabilization, centrifugation, drying, filtration, incineration, wet oxidation,
and disposal in landfill or on soil.
Typical Activated Sludge Treatment Plant:

Unit Operations in Wastewater Treatment:
Industrial
wastewaters typically contain a number of heavy metals, halogenated organic
compounds, and other priority pollutants. Therefore, industrial facilities must
process their wastewaters before discharging to either a body of water (under
NPDES permit) or a POTW.
Typically,
these pollutants are removed from the wastewater using a variety of pretreatment processes, especially physical and chemical processes.
Examples
include coagulation, flocculation precipitation, sedimentation, filtration, ion
exchange, air stripping, membrane separation activated carbon adsorption, wet
oxidation and photochemical oxidation.
Example
of Physical-Chemical Wastewater Treatment Process:
Typical
Physical-Chemical Treatment Plant:
Physical
Separation Processes:
Activated carbon adsorption
Distillation
Electrolytic recovery
Hydrolysis
Ion exchange
Solvent extraction
Membrane separation technologies
Air stripping and steam stripping
Thin film evaporation
Freeze-crystallization
Operations
Involved in the Removal of Suspended Solid in Wastewaters:
Screening
and comminution
Grit
removal
Sedimentation
Floatation
Filtration
and centrifugation
Coagulation/sedimentation
Operations
Involved in the Removal of Biodegradable Material in Wastewaters:
Activated
sludge treatment
Trickling filters
Rotating biological contactors (RBC)
Aerated lagoons
Anaerobic lagoons
Facultative lagoons
Anaerobic treatment
Operations
Involved in the Removal of VOCs in Wastewaters:
Adsorption
Absorption
Air
stripping
Condensation
Freezing
Incineration
Combustion
Operations
Involved in the Removal of Nitrogen in Wastewaters:
Biological
nitrogen utilization in activated sludge process
Biological
nitrification and denitrification
Air stripping of ammonia
Chlorination
Adsorption
Operations
Involved in the Removal of Phosphorus in Wastewaters:
Biological
phosphorus utilization in activated sludge process
Chemical
additions (metal salts or polymers)
Lime
addition
Biological/chemical
treatment
Operations
Involved in the Removal of Organic Priority Pollutants in Wastewaters:
Aerobic
biological treatment
Anaerobic
biological treatment
Biological
treatment/activated carbon adsorption (PACT)
Adsorption
Chemical
oxidation
Operations
Involved in the Removal of Heavy Metals and Dissolved Inorganic Solids in
Wastewaters:
Chemical
oxidation/reduction and precipitation
Ion
exchange
Reverse
osmosis
Ultrafiltration
Electrodialysis
Example
of Heavy Metal Remova :
Example
of Organic Chemical Removal:
Classification
of Hazardous Waste Treatment Processes:
Physical
Chemical
Biological
(Disposal)
Physical
and Chemical Processes for Hazardous Waste Treatment:
Filtration and separation
Chemical precipitation
Photolysis
Chemical oxidation and reduction
Dehalogenation
Ozonation
Evaporation
Solidification and stabilization
Biological
Processes for Hazardous Waste Treatment:
Aerobic processes
Anaerobic
digestion
Composting of industrial wastes
Land treatment of industrial wastes
Biodegradation of environmental pollutants
Enzymatic systems
Thermal
Processes for Hazardous Waste Treatment:
Liquid injection incineration
Rotary kilns
Fluidized-bed
thermal oxidation
Hazardous wastes as fuel for burners
Cement kilns
Wet oxidation
Pyrolisis processes
Oceanic incineration
Molten glass processes
Deep shaft wet air oxidation
Supercritical water extraction
Plasma systems
Mobile thermal treatment systems
Catalytic incineration
Infectious waste incineration
Land
Storage and Disposal of Hazardous Wastes:
Surface impoundment
Disposal in mines and sand domes
Aboveground disposal
Hazardous waste landfilling
Subsurface injection of liquid hazardous wastes
Additional
Information and Examples on Wastewater and Waste Treatment Processes
References:
Droste,
R. L., 1997, Theory and Practice of Water and Wastewater
Treatment,
John Wiley & Sons, New York
Eckenfelder,
W. W., Jr., 1989, Industrial Water Pollution Control,
LaGrega,
M. D., Buckingham, P. L., Evans, J. C., 1994, Hazardous
Waste
Management, McGraw-Hill , New York
Metcalf
& Eddy, 1991, Wastewater Engineering: Treatment, Disposal,
and
Reuse, McGraw-Hill , New York
Sundstrom,
D. W. and Klei, H. E., 1979, Wastewater Treatment,
Prentice
Hall, Englewood
Wentz,
C. W., 1995, Hazardous Waste Management, 2nd edition, McGraw-Hill, New
York, pp. 153 - 248.