Introduction:
Introduction:
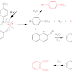
Cellulosic fiber-containing fabrics are made wrinkle resistant by a durable press wrinkle-free process which comprises treating a cellulosic fiber-containing fabric with formaldehyde, a catalyst capable of catalyzing the crosslinking reaction between the formaldehyde and cellulose and a silicone elastomer, heat-curing the treated cellulose fiber-containing fabric, preferably having a moisture content of more than 20% by weight, under conditions at which formaldehyde reacts with cellulose in the presence of the catalyst without a substantial loss of formaldehyde before the reaction of the formaldehyde with cellulose to improve the wrinkle resistance of the fabric in the presence of a silicone elastomeric softener to provide higher wrinkle resistance, and better tear strength after washing, with less treatment.
Types of Wrinkle Free Process of Cotton Shirts:
I guess the idea of a shirt which has not to be ironed anymore is as old as the cotton shirt itself. Apart from all attempts which has started decades ago to establish a shirt of Polyester or any other artificial fibre failed, as the consumer understood from the beginning the positive attributes of the cotton fibre. By its ability to hold moisture and to release it controlled, cotton is one of the most ideal fibres among all. It is breathable and remains a good feeling for the garment bearer. This may has changed by today´s new developments of artificial fibres, which have nothing to do with those from the sixties of the last century. But still the cotton fibre is number one in peoples mind when they think of comfort. In the last decade new technologies have been established to prepare the cotton fabric by chemicals, to make them almost wrinkle free or, as some manufacturer call it wrinkle resistant (WR). Basically four different technologies are known today to do so.
• Pre- Curing
• Post- Curing
• Dip- Spin
• Vapor – Phase
But, what basically happens during or after this process ?
All four systems have one issue in common, the cotton fibre is swelled artificially and by this process it is loosing its memory. Instead of being curled as it naturally is, it becomes straight. And as it has increased its diameter, it is almost impossible to crease. The negative aspect is, that by this process it looses a part of its tensile strength and habit to absorb moisture.
Pre- Cured fabric:
Fabric can be a 100 % cotton fabric or cotton blend.Contrary to all the other WR processes, by this system the fabric does not need any further heat treatment as the curing process has been done already before the shirt is manufactured. The already finished fabric is resistant to wrinkles already. Unfortunately no crisp and sharp creases can be realist for collars, cuffs and front placket edges. As the fabric does not accept any final pressing.Only a shirt finisher with steam and air is required.
Post- Cured fabric:
In this case the fabric can be a 100 % cotton fabric or a cotton blend. The fabric will be delivered with the curing chemical inside. The roll of fabric is sealed in a polyester bag . Once the bag is opened the fabric has to be manufactured entirely, as it cannot be stored for a long time. After the shirt is manufactured, it has to be pressed entirely. After it has been put on a hanger it will be cured in a hanging position on a cloth rack inside an oven for 3- 5 min. by about 130°C to 150”C (depends on the chemical used).
Now the shirt is ready for folding and bagging.
Dip- Spin system:
This one belongs to the most popular process for wrinkle free shirts and can be used for 100 % cotton fabrics or cotton blends. After the shirt is manufactured as usually, it will be dipped into a mixture of chemicals, which will be absorbed by the cotton fibres. After the treatment in a tumbler the shirt is still moisturized and has to be pressed entirely. Important is, that during the pressing operation on the various Veit- Kannegiesser Collar-, Cuff- and Body- Presses, the curing process will start already. After pressing the shirt will be put on a hanger and can be cured in a curing oven by about 140°C for about 3 -5 min. One of the key factors for a perfect appearance is the pressing quality, as after the curing operation in an oven, all wrinkles will stay for life. A re-touching by an iron is impossible.
Vapor – Phase:
This curing system can be used in some countries only as very aggressive chemicals are used. Similar to the DIP SPIN system the shirt is manufactured as usually. After the final pressing, a special curing oven is used as instead of liquid chemicals, gas is used to make the shirt resistant to wrinkles. The gas is circulating through the oven and penetrates into the cotton fibre. After a while the gas has to be evacuated from the oven. Before the shirt is folded and bagged, it needs to be washed in order to remove left chemicals inside.




 Spinning- Yarn manufacture:
Spinning- Yarn manufacture: Racks of bobbins are set up to hold the thread while it is rolled onto the warp bar of a loom. Because the thread is fine, often three of these would be combined to get the desired thread count.[citation needed].
Racks of bobbins are set up to hold the thread while it is rolled onto the warp bar of a loom. Because the thread is fine, often three of these would be combined to get the desired thread count.[citation needed]. Knitting by machine is done in two different ways; warp and weft. Weft knitting (as seen in the pictures) is similar in method to hand knitting with stitches all connected to each other horizontally. Various weft machines can be configured to produce textiles from a single spool of yarn or multiple spools depending on the size of the machine cylinder (where the needles are bedded). In a warp knit there are many pieces of yarn and there are vertical chains, zigzagged together by crossing the yarn.
Knitting by machine is done in two different ways; warp and weft. Weft knitting (as seen in the pictures) is similar in method to hand knitting with stitches all connected to each other horizontally. Various weft machines can be configured to produce textiles from a single spool of yarn or multiple spools depending on the size of the machine cylinder (where the needles are bedded). In a warp knit there are many pieces of yarn and there are vertical chains, zigzagged together by crossing the yarn.





 Copper rivets for reinforcing pockets are a characteristic feature of blue jeans.
Copper rivets for reinforcing pockets are a characteristic feature of blue jeans.